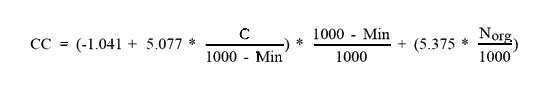3.03
2.60
2.48
2.12
1.22
1.09
0.91
0.00
|
So, with these numbers it only requires determination of the concentration of these groups of compounds! How to do that? Preferably by using rather old methods, which are designed to measure a whole group of compounds at the same time. However, before doing that you have to separate a plant (organ) chemically into several fractions. A relatively simple method, is the following: |

|
| This scheme requires to extraction steps, one Bligh & Dyer extraction yielding a soluble
fraction S2 and a residue R2, and a digestion of starch, yielding the fractions S3 and R3.
Most determinations are relatively simple and standard. More information can be found in, e.g.,
Poorter & Villar (1997).
The determination of lignin is explained here.
|
| If you add the estimated concentrations of these eight groups, you should get values close to 100%. However, especially with biomass that contains a lot of secondary compounds, even simple determinations may get fooled. Depending on organ and species, you may come within 5% of total recovery. However, in some cases you may miss out on 10 - 20%. You would not like to miss out on CC just because your determinations missed out on a part. One solution is to assume that the missing part has similar chemical composition as the part that is known. An alternative is to assume that this missing gap was (hemi)-cellulose, as this is very easily overlooked. |